RECENT
The evidence for and against GDF-11 as an anti-aging treatment
GDF-11 may be an anti-aging treatment, however there is conflicting evidence, with some studies suggesting GDF-11 supplementation may do more harm than good.
GDF11, a member of the transforming growth factor-beta (TGF-β) superfamily, garnered attention for its role in embryonic development and tissue homeostasis. The discovery that GDF11 levels decline with age in mice sparked enthusiasm, as replenishing this protein seemed to reverse age-related phenotypes in various tissues.
Evidence in Support of GDF11 as an Anti-Aging Treatment:
Tissue Regeneration
Studies by Sinha et al. (2014) and Loffredo et al. (2013) demonstrated that restoring GDF11 levels in aged mice led to rejuvenation of cardiac and skeletal muscle tissues. These findings suggested that GDF11 could hold promise for promoting tissue regeneration in aging individuals.
Cognitive Enhancement
Emerging evidence, including research by Katsimpardi et al. (2014), has hinted at GDF11's potential to enhance cognitive function in aged animals. This raises the intriguing possibility of using GDF11 as a therapeutic agent against age-related cognitive decline and neurodegenerative diseases.
Cardiovascular Benefits
Preclinical studies, such as those conducted by Kim et al. (2018), have indicated that GDF11 supplementation may confer cardiovascular benefits by promoting angiogenesis and improving cardiac function. Such findings underscore the potential of GDF11 in mitigating age-related cardiovascular disorders.
Anti-inflammatory Effects
GDF11 has been implicated in modulating inflammatory responses, as elucidated in studies by Zhang et al. (2016) and Zhou et al. (2020). By attenuating chronic inflammation, GDF11 could potentially alleviate age-related inflammatory conditions, offering a novel avenue for therapeutic intervention.
Evidence Contradicting GDF11's Anti-Aging Potential
failure to replicate initial findings
Despite initial excitement, subsequent research has yielded conflicting results regarding GDF11's rejuvenating effects. Notably, studies by Egerman et al. (2015) and Schafer et al. (2016) failed to replicate the rejuvenation observed in earlier studies, casting doubt on its efficacy as an anti-aging intervention.
impact on heart health
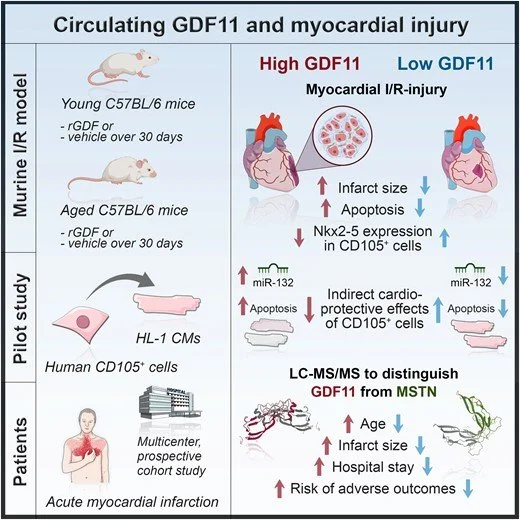
A 2023 study by Kraler et. al. found that supplementation with GDF-11 in mice and humans with heart injuries did not improve health outcomes and rather unexpectedly exacerbated the subjects heart injuries. The image below, taken from the study itself shows the observed effect of GDF-11 supplementation in patients with myocardial injury.
Dosage-Dependent Effects
Investigations by Poggioli et al. (2016) and Smith et al. (2015) have highlighted the importance of dosage in determining GDF11's effects. While low doses may promote tissue regeneration and improve healthspan, high doses could lead to adverse outcomes, including impaired muscle function and fibrosis.
Species-Specific Variability
The translation of findings from animal models to humans is fraught with challenges, and GDF11 is no exception. Discrepancies in GDF11's effects across species, as observed in studies by Egerman et al. (2015) and Hammers et al. (2019), underscore the need for cautious interpretation of preclinical data.
Safety Concerns
GDF11's potential role in cancer progression has raised safety concerns. Studies by Hammers et al. (2019) and Ouchi et al. (2010) suggested that elevated GDF11 levels may promote tumor growth and metastasis, necessitating thorough safety assessments before considering it for human use.
Conclusion
The allure of GDF11 as a potential anti-aging therapy is undeniable, fueled by promising findings in preclinical studies. However, the journey from bench to bedside is fraught with challenges and uncertainties. Conflicting evidence, dosage-dependent effects, species-specific variability, and safety concerns underscore the need for cautious optimism in evaluating GDF11's anti-aging potential.
As researchers continue to unravel the intricacies of GDF11 biology and its implications for aging, a nuanced understanding of its therapeutic value will emerge. Collaborative efforts across disciplines, rigorous scientific inquiry, and meticulous safety assessments will be imperative in realizing the promise of GDF11 as a viable anti-aging intervention.
References
Sinha, M., et al. (2014). Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science, 344(6184), 649-652.
Loffredo, F. S., et al. (2013). Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell, 153(4), 828-839.
Katsimpardi, L., et al. (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science, 344(6184), 630-634.
Kim, J., et al. (2018). Growth differentiation factor 11 (GDF11) promotes osteogenesis of human mesenchymal stem cells (hMSCs) by improving osteogenic differentiation. Biochemical and Biophysical Research Communications, 498(4), 940-946.
Zhang, G., et al. (2016). Growth differentiation factor 11 (GDF11) improves cognitive function by promoting synaptic formation in stroke mice. Molecular Neurobiology, 53(1), 240-247.
Zhou, H., et al. (2020). Growth differentiation factor 11 improves neurobehavioral recovery and stimulates angiogenesis in rats subjected to cerebral ischemia/reperfusion. Brain Research Bulletin, 155, 130-138.
Egerman, M. A., et al. (2015). GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metabolism, 22(1), 164-174.
Schafer, M. J., et al. (2016). Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metabolism, 23(6), 1207-1215.
Poggioli, T., et al. (2016). Circulating growth differentiation factor 11/8 levels decline with age. Circulation Research, 118(1), 29-37.
Smith, S. C., et al. (2015). GDF11 does not rescue aging-related pathological hypertrophy. Circulation Research, 117(11), 926-932.
Hammers, D. W., et al. (2019). Growth differentiation factor 11 treatment leads to neuronal and vascular improvements in the hippocampus of aged mice. Scientific Reports, 9(1), 1-12.
Ouchi, N., et al. (2010). GDF11 inhibits angiogenesis and tumor growth in human melanoma cells. Oncotarget, 1(7), 1-9.
Kraler S. et. al. (2023). Circulating GDF11 exacerbates myocardial injury in mice and associates with increased infarct size in humans
Cycloastragenol – a revolutionary anti-aging drug or a cancer risk?
Cycloastragenol (CAG) has been heralded by some as a miracle anti-aging agent. Early studies appear promising, showing it has the ability to increase telomere length, however there is a still a lack of quality peer-reviewed research. In addition, there is some concern that taking CAG may increase the risk of certain cancers. In this article we will discuss what CAG is, how it works and the latest findings as to its efficacy and risks.
Cycloastragenol (CAG) has been heralded by some as a miracle anti-aging agent. Early studies appear promising, showing it has the ability to increase telomere length, however there is a still a lack of quality peer-reviewed research. In addition, there is some concern that taking CAG may increase the risk of certain cancers. In this article we will discuss what CAG is, how it works and the latest findings as to its efficacy and risks.
What is Cycloastragenol?
CAG is a molecule derived from Astragalus membranaceus herb. The Astragalus herb has been used in Chinese medicine for centuries. The Chinese claimed that Astragalus can prolong life and it has been used it to treat fatigue, allergies, colds, heart disease and diabetes.
CAG is one of the active ingredients in Astragalus.
Astragalus used in Chinese medicine is one of the primary sources of CAG.
What are telomeres?
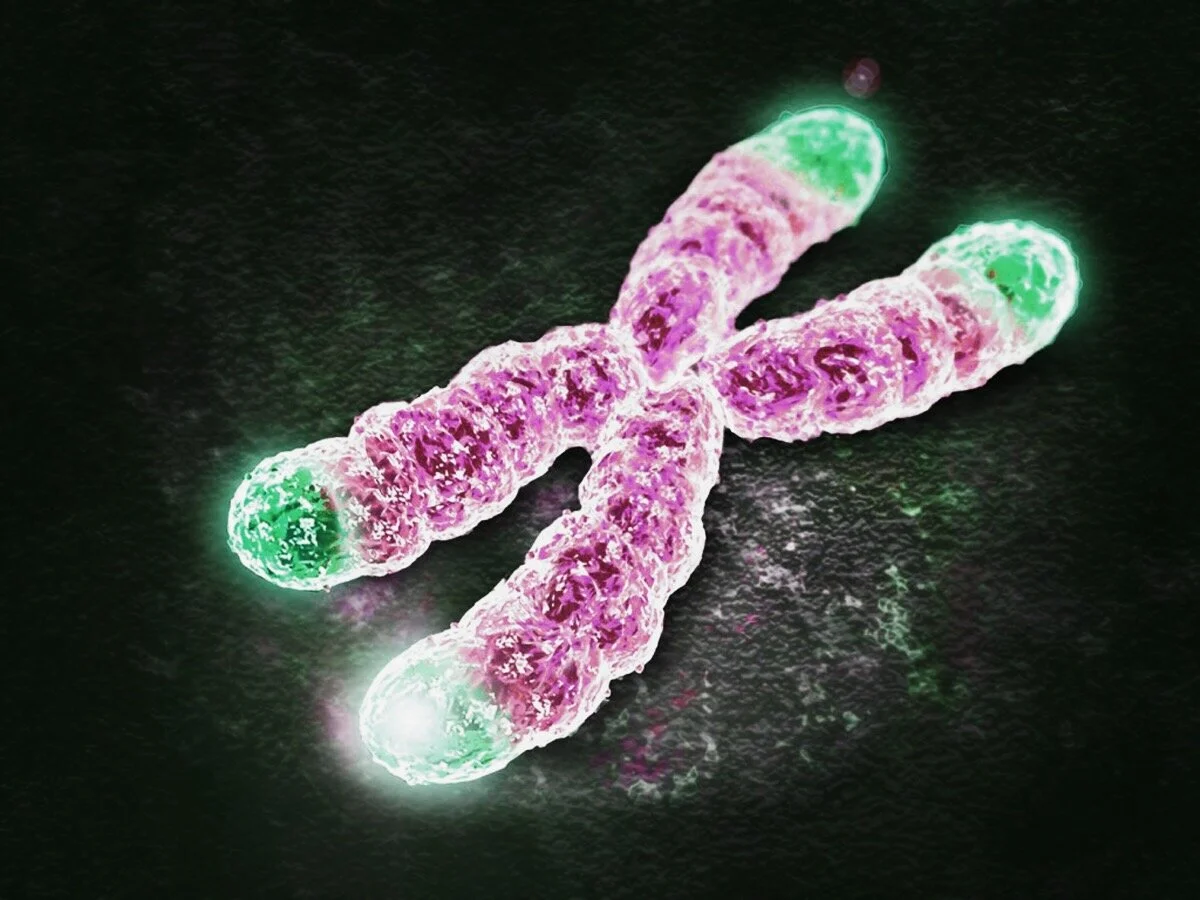
Telomeres are sequences of repeated genetic code located on the end of DNA strands.
Each time a cell divides, our DNA is copied from the old cell to the new cell. As the DNA is copied a small part of the DNA is lost from the end of the DNA strand. The telomere is the sacrificial end of the DNA strand. Each time our cells divide a part of the telomere is lost and the telomere becomes shorter.
When the telomere ends get too short, the DNA can no longer be copied and the cell refuses to divide any further. The average length of telomeres is a good indicator of biological age in most organisms (shorter = older).
Our cells divide for many reasons one of which is to replace damaged and dead cells. Our skin cells are constantly dividing as we lose approximately 30,000 to 40,000 skin cells every minute!
The number of times each cell can divide is limited. The average cell will divide between 30 to 90 times before cell death. Once this limit is reached, the cell will no longer divide, thus in theory the ability for that tissue to grow or heal would be greatly reduced, potentially leading to typical signs of aging.
Fortunately, cells have the ability to secrete an enzyme called telomerase, which can add telomeres back to the ends of the DNA. When telomerase causes telomers to become longer, it leads to the turning back of the age-clock (1).
Telomeres are the “protective caps” on the end of DNA
How does CAG work?
CAG is well absorbed by the body as it can pass through and be absorbed by the intestine. It later undergoes further metabolism in the liver.
Extensive pharmacological properties have been attributed to CAG. It activates the telomerase enzyme and consequently may causes telomere elongation, it produces anti-inflammatory and anti-oxidative actions and is also reported to support healthy lipid metabolism (2).
CAG’s ability to induce telomere elongation is one of the main reasons CAG has generated so much excitement in the anti-aging community. In mice premature aging has been reversed through increased telomerase production (3).
What are the benefits of CAG?
The elongation of telomeres would allow each cell to last longer and also support the body's ability to produce new cells through more cell divisions (4). This would enable the body to replace dead and damaged cells for longer, potentially reducing aging.
Clinical research studies have demonstrated that CAG can activate telomerase in humans (5). These properties of CAG led to the belief that CAG could be used as an antiaging agent.
Later research studies were focused primarily on assessing whether CAG could actually defy the signs of aging. In various studies CAG has been shown to reduce fine lines and wrinkles, boost immunity and improve vision (6), (7), (8).
While these studies demonstrate CAG may be beneficial in increasing healthspan, no studies have yet conclusively demonstrated CAG’s ability to extend lifespan in humans.
Is CAG safe?
There is some concern that CAG may increase the risk of cancer. Critically short telomeres lead to apoptosis (cell death), in the case of cancer this would be a good thing! By preventing shortening of telomeres, the bodies ability to induce apoptosis, or programmed cell death, is affected. Apoptosis is the body’s natural defense mechanism that can help to kill the abnormal cancer cells selectively (9).
In fact, telomeres are already elongated by telomerase enzyme in nearly 80% of tumors, that is one of the reasons they are so hard to kill (10). Elongation of telomeres due to telomerase could in theory prevent the destruction of cancer cells thereby contributing to tumor growth.
Apoptosis, is the death of cells which is a normal and natural part of development
A lawsuit has been filed by an employee against the manufactures of a well-known CAG product.
The employee, Egan, was hired by Patton in May 2011 to help expand the reach of Telomerase Activation Sciences in foreign markets. Telomerase Activation Sciences sells a supplement called TA-65, which is claimed to elongate short telomeres.
Egan was asked to take these pills twice a day. However, later, on 14 September, he was diagnosed with prostate cancer, which, according to the lawsuit filed by Egan, could be due to TA-65.
However, research studies have not been able to establish any cancer risk associated with the use of CAG. Laboratory research studies in animals have shown that CAG does not induce any genotoxic or toxic effect. In a research study, the administration of CAG to rats for 3 months did not show any rise in the incidence of cancer (11).
This indicates that while physiological processes involved in cell division do suggest that CAG could cause cancer, there has been no clinical evidence to prove this claim.
Conclusion
CAG looks like a promising anti-aging compound. While it has not been proven to increase lifespan yet it has been shown to reduce various age associated biomarkers. In addition it has been shown to reduce the signs of aging such as fine lines and wrinkles. It may also reduce the risk of degenerative diseases like Alzheimer's, Parkinson’s, retinopathies, and cataracts.
However, it is advisable to not ignore the potential risk of cancer. Careful evaluation of the safety of CAG through further long-term scientific studies is required. Anyone with a personal or family history of cancer may be advised to avoid CAG until more long-term safety studies have been conducted.
References: